-
Primary Endpoint
-
Secondary Endpoints
![Kaplan-Meier Curve of Progression-Free Survival (PFS) for KYPROLIS + lenalidomide + dexamethasone/KRd (n=396) and lenalidomide + dexamethasone/Rd (n=396).
Proportion surviving without progression from 0 to 100 percent. Months since randomization from 0 to 48 months.
In the intention-to-treat population, 431 events (disease progression or death), based on outcomes assessed by the independent review committee, had occurred (207 events in the KRd group [52.3%]; 224 events in the Rd group [56.6%]).
Patients in the KRd arm demonstrated improved PFS compared with those in the Rd arm (HR = 0.69 [95% CI: 0.57–0.83], p<0.0001); with 26.3 months in the KRd arm vs. 17.6 months in the Rd arm).
Patients at risk for Rd: 396 at Month 0, 287 at Month 6, 206 at Month 12, 151 at Month 18, 117 at Month 24, 72 at Month 30, 18 at Month 36, 1 at Month 42, and 0 at Month 48.
Patients at risk for KRd: 396 at Month 0, 332 at Month 6, 279 at Month 12, 222 at Month 18, 179 at Month 24, 112 at Month 30, 24 at Month 36, 1 at Month 42, and 0 at Month 48.
Patients received KYPROLIS (carfilzomib) through to cycle 18.](-/media/Themes/Amgen/Amgencompass-ca/Amgencompass-Kyprolis/images/english/efficacy-data/aspire-study/ed-aspire-study-pfs-graph.jpg)
Adapted from the KYPROLIS Product Monograph1 and Stewart et al.9
| Subgroup | KRd | Rd | |
|---|---|---|---|
| 1 prior line of therapy10 | Median PFS (months) | 29.6 | 17.6 |
| Progression or death events, n/N | 91/184 | 88/157 | |
| HR=0.71 [95% CI: 0.53–0.96] p=0.0118; one-sided | |||
| ≥2 prior lines of Therapy10 | Median PFS (months) | 25.8 | 16.7 |
| Progression or death events, n/N | 116/212 | 136/239 | |
| HR=0.72 [95% CI: 0.56–0.92] p=0.0046; one-sided | |||
| High cytogenetic risk11† | Median PFS (months) | 23.1 | 13.9 |
| Progression or death events, n/N | 31/48 | 32/52 | |
| HR=0.70 [95% CI: 0.43–1.16] p=0.0829; one-sided | |||
| Standard cytogenetic risk11† | Median PFS (months) | 29.6 | 19.5 |
| Progression or death events, n/N | 68/147 | 94/170 | |
| HR=0.66 [95% CI: 0.48–0.90] p=0.0039; one-sided | |||
Open label trial; pre-planned subgroup analysis; results were not adjusted for multiplicity and should be interpreted descriptively.10,11
![Kaplan-Meier Curve of Overall Survival (OS) for KYPROLIS + lenalidomide + dexamethasone/KRd (n=396) and lenalidomide + dexamethasone/Rd (n=396).
Proportion surviving from 0 to 100 percent. Months since randomization from 0 to 78 months.
The pre-planned OS analysis was performed after 246 deaths in the KRd arm (62.1%) and 267 deaths in the Rd arm (67.4%).
A statistically significant advantage in OS was observed in patients in the KRd arm compared to patients in the Rd arm (HR = 0.79 [95% CI: 0.67–0.95; p=0.0045]), with 48.3 months in the KRd arm vs. 40.4 months in the Rd arm.
Patients at risk for Rd: 396 at Month 0, 356 at Month 6, 313 at Month 12, 281 at Month 18, 243 at Month 24, 220 at Month 30, 199 at Month 36, 176 at Month 42, 149 at Month 48, 133 at Month 54, 113 at Month 60, 69 at Month 66, 20 at Month 72, and 3 at Month 78.
Patients at risk for KRd: 396 at Month 0, 369 at Month 6, 343 at Month 12, 316 at Month 18, 282 at Month 24, 259 at Month 30, 232 at Month 36, 211 at Month 42, 190 at Month 48, 166 at Month 54, 149 at Month 60, 88 at Month 66, 22 at Month 72, and 0 at Month 78.
Patients received KYPROLIS through to cycle 18.](-/media/Themes/Amgen/Amgencompass-ca/Amgencompass-Kyprolis/images/english/efficacy-data/aspire-study/ed-aspire-study-pfs-graph3.jpg)
Adapted from Siegel et al.12
- The median OS improved by 7.9 months in patients in the KRd arm compared with those in the Rd arm1
- The pre-planned OS analysis was performed after 246 deaths in the KRd arm and 267 deaths in the Rd arm1,12
- The median follow-up was approximately 67.1 months1,12
- 28.6 months [95% CI: 24.9–31.3] in the KRd arm, vs
- 21.2 months [95% CI: 16.7––25.8] in the Rd arm
CHARACTERISTICS
* KYPROLIS treatment was administered for a maximum of 18 cycles unless discontinued early for disease progression or unacceptable toxicity. Lenalidomide and dexamethasone administration could continue until progression or unacceptable toxicity.1
† Percentages have been rounded.
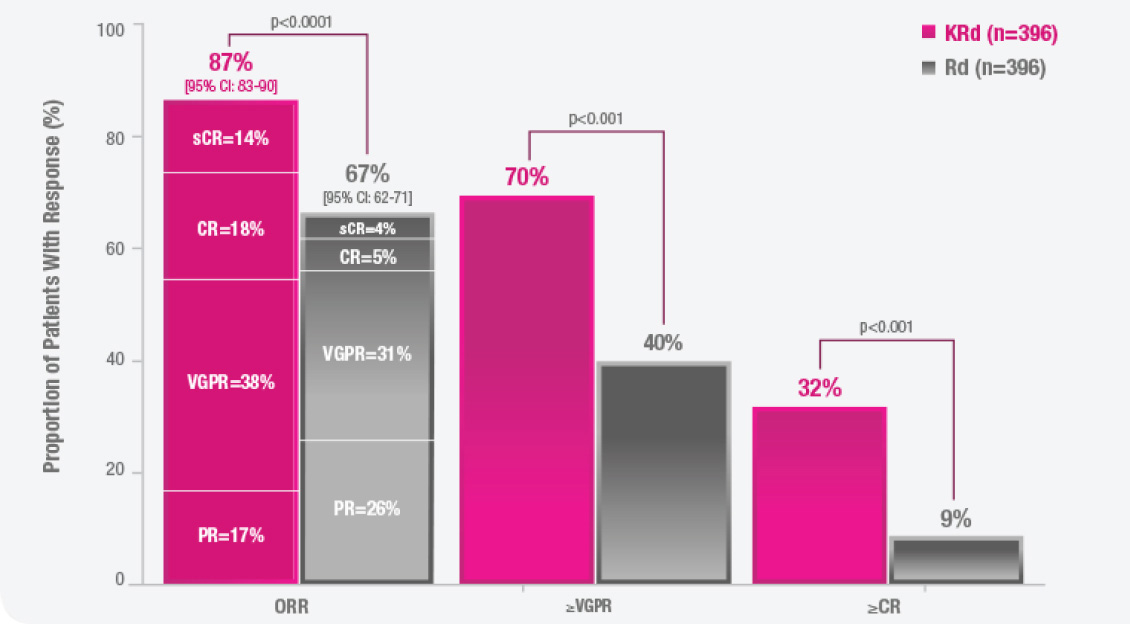
CI, confidence interval; CR, complete response; HR, hazard ratio; KRd, KYPROLIS + lenalidomide + dexamethasone; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; sCR, stringent complete response; Rd, lenalidomide + dexamethasone; VGPR, very good partial response.



